How To Search the Chemical Makeup of Exoplanet Atmospheres for Hints at Their History
By Andy Tomaswick
Author’s note – this article was written with Dr. Vincent Kofman, a scientist at NASA’s Goddard Space Flight Center (GSFC), and the lead author on the research it discusses.
Thousands of exoplanets have been discovered in the recent decades. Planet hunters like TESS and Kepler, as well as numerous ground-based efforts, have pushed the field and we are starting to get a total number of planets that will allow us to perform effective statistical analysis on some of them.
Not only do the detected number of planets show us how common they are; it exposes our lack of understanding about how planets form, what conditions are present, and when planets may be habitable. The transit detection of an exoplanet primarily yields the orbital period, or the length of a year on the planet, and the relative size of the planet with respect to the star. The next steps are to characterize the planet. This usually requires follow up studies, using different observational strategies and more powerful telescopes.
Next to studying the occurrence, sizes, orbital periods, and the amount of light they receive, the composition of the atmospheres can provide much insight into our understanding of these new worlds. The composition of the atmospheres of exoplanets can be revealed by observing these using space-based telescopes, such as the Hubble Space Telescope, or from the ground using observatories like the Very Large Telescope or Keck.
These remote observations rely on interaction of the molecules in the atmosphere with light, and are highly specific to the conditions in the atmosphere, serving as a strong diagnostic for both the planet’s composition and its temperature. However, not all molecules are equally visible and the light from exoplanets very faint. Therefore, currently we are only able to see the brightest molecules, such as water, methane, carbon monoxide, sodium, as well as a number of metal-oxides. For the rest of the atmosphere, the planets in our solar system provide a first start to what may be present, but scientists strongly rely on chemical and physical models to assess what may be hidden from their spectroscopic studies.

Fortunately, the detectable molecules can teach us many things about the conditions in the atmosphere. For instance, the carbon to oxygen (C/O) ratio, inferred from the abundance of (amongst others) carbon monoxide, carbon dioxide, methane, and water, essentially indicates whether the chemistry in the atmosphere is oxygen or carbon dominated. These are different chemical end members, and lead to very different environments. Titan’s atmosphere for instance is carbon dominated, leading to a hazy world with hydrocarbon lakes. Mars’ atmosphere is an example of a C/O ratio of less than 1. As the C/O ratio can also be determined in protoplanetary disks, this is a valuable ratio that may link the birthplace of planets to their current state.
Another stochiometric ratio that has proven to be very insightful in the solar system, is that of hydrogen (H), the most common element in the universe, to its slightly heavier isotope, deuterium (D). Known as the D/H ratio, it can provide a glimpse into the history and planet and its atmosphere, and is the focus of a new paper from scientists at NASA’s Goddard Space Flight Center (GSFC), led by Dr. Vincent Kofman.
The D/H ratio was originally set as part of the Big Bang at about 1 / 8700 – or 8700 atoms of hydrogen for every one of deuterium. There are not many natural processes that have changed that ratio over time, with the exception of some active processes in stars. That 1/8700 ratio is then passed on to planets as they begin to form, yet the initial endowment value can differ across the formation region in the nebula, where stars and planets form. This is because of the different temperatures at which hydrogen and deuterium containing molecules freeze out. Particularly for the extremely cold regions, the amount of deuterium is substantially higher. Planets can therefore have very different primordial D/H values depending on when and how they form. Our solar system is a good example where that original ratio was in place during the planetary formation process.
The higher deuterium content of primordial ices is which is why the ice giant Uranus and Neptune have a higher D/H ratio than Jupiter and Saturn. After the planets were formed, though, the ratio on some planets changed. For the rocky planets, it is believed that they received their water from asteroids and comets, which formed at very different locations in the nebula as those planets, resulting in higher deuterium content in the atmospheres of Earth, Venus, and Mars.
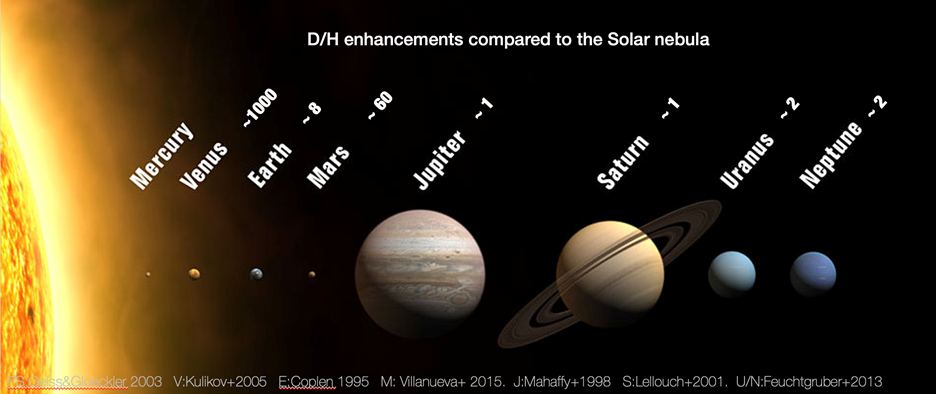
Credit: NASA / Kofman
Subsequently, that ratio was increased even more by significant water loss. This effect, which can be most starkly seen on Mars and Venus, can be understood as following. As much of the hydrogen and deuterium in planetary atmospheres is tied up in water, which is easily destroyed by sunlight, resulting in elemental oxygen and hydrogen.
That hydrogen, floating high in the atmosphere, is then susceptible to being accelerated into space by the solar wind, then flying fast enough to escape the gravity of the terrestrial planets. With that loss of hydrogen, the water molecule cannot reform, and the planet is left with a lower total quantity of water. Over the course of billions of years, this process, if it continues, can cause a significant drop in the water content of a planet’s atmosphere.
However, there is one confounding factor in this story of lost water – deuterium, which is approximately twice as heavy as elemental hydrogen, is much less likely to be blown into space. Therefore, any “heavy” water molecule that is split in the atmosphere is much less likely to lose its deuterium atom than a normal water molecule is to lose its regular hydrogen atom. Over billions of years, this increases the D/H ratio in those atmospheres.
To be able to investigate the D/H ratio on exoplanets the GSFC researchers had to pull information from huge spectroscopic databases. In order to lessen the burden, they built a tool that allowed them to do so orders of magnitudes more quickly than existing systems. The databases have been incorporated into a tool they built called the Planetary Spectrum Generator (PSG). PSG is an online tool that allows the simulation spectra of (exo)planets, taking into consideration all elements of the calculations (the Solar/stellar spectrum, the planets’ surface and atmosphere, as well as absorption by the Earth’s atmosphere and the specifics of the telescope used).
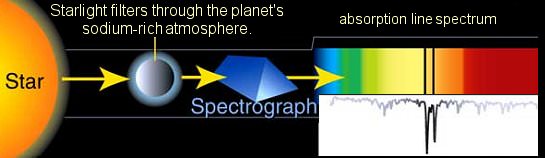
Image Credit: A. Feild, STScl, NASA
Using the Planetary Spectrum Generator to simulate the interaction of the exoplanet Trappist 1b with the light of its star while passing in front of it, the researchers have investigated the possibility of detecting the D/H ratio using the soon-to-be-launched James Webb Space Telescope. They demonstrated that for atmospheres rich in water, the D/H ratio could be constrained by observing a few transits of the planet in front of its host star.
With a better understanding of the D / H ratio, exoplanet hunters should be able to determine some of the atmospheric and hydrological history of these new planets. This will improve our understanding of the chemistry taking place on exoplanets and refine atmospheric models. Ultimately, it may enable a better grip on what it takes for a planet to be habitable.
Learn More:
Journal of Quantitative Spectroscopy and Radiative Transfer – Absorption in exoplanet atmospheres: Combining experimental and theoretical databases to facilitate calculations of the molecular opacities of water
Planetary Spectrum Generator
Philosophical Transactions of the Royal Society – D/H ratios of the inner Solar System
UT – The Color of Habitable Worlds
UT – New Technique to Search for Life, Whether or not it’s Similar to Earth Life
Lead Image
Artist’s conception of the Trappist system.
Credit – NASA / JPL-Cal Tech
The post How To Search the Chemical Makeup of Exoplanet Atmospheres for Hints at Their History appeared first on Universe Today.

June 13, 2021 at 07:07PM
via Universe Today read more...

Post a Comment